
Department of Chemistry, SUNY-Potsdam Slide 1 2 3 4 5 6 7 8 9 10
Presented at: <<< >>>
The National Meeting of the American Chemical Society
Philadelphia, PA, August 18-22, 2008


| Symposium: Fundamental Research in Colloid and Surface Chemistry | |
|
"Interactions of toxic heavy metal ions with glutathione self-assembled monolayer films", M. Hepel, J. Dallas, Department of Chemistry, State University of New York at Potsdam, Potsdam, NY |
|
| Abstract | |
| The redox regulating glutathione (GSH) system plays a vital role in living organisms in protecting cells against oxidative damage. There are indications that susceptibilities of different individuals to environmentally induced diseases (diabetes, brain damage and cancer) are associated with reduced GSH concentration levels. The interactions of GSH with toxicants, including heavy metal ions, are suspected to influence the regulating capacity of GSH/GSSG system. We have found that the modification of a Au substrate with self-assembled glutathione film creates a framework of confined-space microenvironment with ion-channels for enhanced Hg2+-GSH interactions and charge transfer reactivity. The chelation of Hg2+ to carboxylate moieties of Au-SG and place–exchange of Hg/Au atoms have been further investigated by ab initio quantum mechanical calculations. The electronic structure of Hg2+-GSH chelate was found to mimic that of Ca2+-GSH. The implications of this finding for studies of environmental effects on susceptibility to degenerative diseases will be discussed. | |
 |
|
|
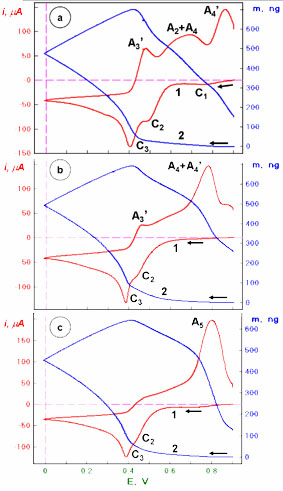
Fig. 1. Cyclovoltammetric (red) and nanogravimetric (blue) permeability characteristics of Hg(II) piezosensors: (a) QC|Au, (b) QC|Au|cysteamine SAM; (c) QC|Au|cysteamine-GSH in 1.5. mM Hg(II) solution. |
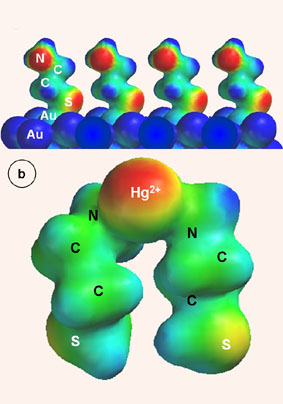
Fig. 2. Electron density surfaces for cysteamine-SAM (CA) on Au piezoelectrode (a) and Hg(CA)2 surface complex (b). Fig. 3. CA-GSH adsorbed on Au cluster in upright (a) and hydrogen-bonded (b) configuration. |