Department of Chemistry, SUNY-Potsdam
Slide 1
2 3 4 5 6 7 8 9 10
Presented at:
<<< >>>
The National Meeting of
the Electrochemical Society
Denver, CO, May
7-12, 2006
Symposium: Nanotechnology
Anodic Photooxidation of Remazol Black B Azo Dye on Transition Metal Oxide
Electrodes
Sandra Hazelton and
Maria Hepel,
Department of Chemistry, State University of New York at Potsdam, 44
Pierrepont Ave., Potsdam, NY 13676, Fax: 315-267-3170, hepelmr@potsdam.edu
Abstract.
The dye pollutants discharged into industrial waste water are often toxic,
carcinogenic, mutagenic, or teratogenic in living organisms. Many dyes,
including the diazo dyes, are very stable and are resistant to chemical
degradation as well as to a microbial attack. We have investigated the
degradation kinetics of various azo dye pollutants (such as the Chicago Sky
Blue, Brilliant Cresyl Yellow, Orange II, Naphthol Blue Black, Methylene Blue,
and others) using photoelectrochemical methods. In this work, the anodic
photo-decomposition of Remazol Black B (RBB), was investigated. Previous studies
devoted to RBB degradation, were based on fungal peroxidase-catalyzed
oxidation [1], and photooxidation by UV light in combination with a powerful
oxidant such as H2O2 [2,3]. The RBB degradation in colloidal semi-conductor
suspensions acting as photocatalysts have also been used [4]. We have found that
photo-electrochemical degradation [5] is superior in terms of the degradation speed
in comparison with other methods. We have observed faster degradation rates
using photoelectrocatalysis on solid semiconductor electrodes as compared to the
degradation rates obtained in colloidal photocatalysis systems.
The preparation of semiconductor electrodes was based on
electrochemical deposition of thin semiconductor films on Pt substrates as
described in our previous papers [5,6]. Nanoparticulate films of TiO2, WO3, and
MoO3, with 50-100 nm thickness and an average size of nanocrystallites from 20
to 40 nm., were used in this work as the electrocatalysts for RBB degradation.
The photocatalyst film morphology and crystalline structure
are of paramount importance for the dye degradation effectiveness. Therefore,
the catalyst film morphology was examined using SEM and AFM imaging techniques
and structure determined using X-ray diffraction. The effect of annealing
temperature on performance of these electrocatalysts have been investigated in
the temperature range from room temperature to 600 oC. Typical plot of the
dependence of RBB degradation rate on annealing temperature is shown in Figure
1. The optimum annealing temperature was found to be 450 oC.
The kinetics of RBB degradation process was followed by monitoring the decay of
absorbance maximum at
lm = 597 nm. The decay rate
was strongly dependent on the applied electrode potential and thus can be
controlled by changing the potential. The efficiency of the photoelectrochemical
degradation process depends also on the dye concentration, selection of a
suitable supporting electrolyte, and solution pH.
The mechanism of reactions taking
place during the photodecomposition process of RBB is based on the electron-hole
pair generation in the semiconductor film and heterogeneous oxidation with
participation of valence-band holes. Using the ab-initio SCF Hartree-Fock
calculations of molecular orbitals and electrostatic potential mapping, the
mechanisms of nucleophilic and electrophilic attacks, which prevail in different
media, are elucidated.
Acknowledgements
This work was supported by ACS-PRF grant No. 33190-B5.
References
1. Young L., Yu J., Wat. Res., 31 (1997) 1187-1193.
2. Ince N. H., Wat. Res., 33 (1999) 1080-1084.
3. Yang Y., Wyatt D. T. II, Bahorsky, M., Textile Chemist and Colorist,
30, (1998) 27-35.
4. Reutergrdh L. B., Iangphasuk, M., Chemosphere, 35 (1997)
585-596.
5. Hepel M., Hazelton S., Electrochim. Acta, 50 (2005) 5278.
6. Hepel M., Luo J., Electrochim. Acta, 47 (2001) 729.
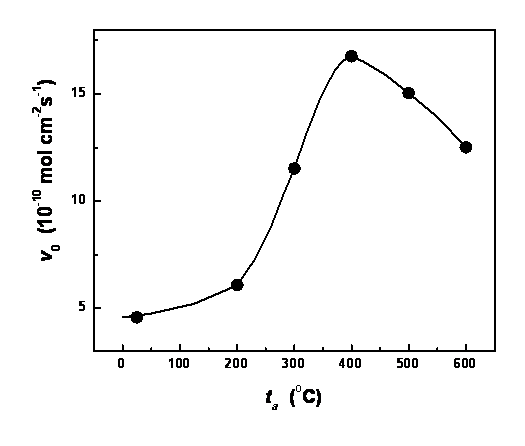
Figure 1. Dependence of
photoelectrocatalytic degradation rate of RBB on the annealing temperature of
n-type WO3 semiconductor film electrode at E = 1.06 V vs. SCE, in a 6x10-5 M RBB solution.
MOTIVATION
Maintaining the natural human habitat in the era
of global industrialization requires immense research efforts to develop
pollution-free technologies and advise efficient methods for cleaning, already
polluted, environment. Many industrial processes release every day tons of
toxic, carcinogenic, and degenerative chemicals. Some of the pollutants remain
in the environment for long time. One of the ubiquitous environmental pollutants
are dyes used in textile and other industries. The textile dyes do not
degrade easily. The dye degradation schemes have been widely investigated and, in recent years, considerable improvement in the degradation
efficiency has been achieved. It has been reported that several organic
pollutants can be degraded on illuminated semiconductor powders. On the other
hand, photoelectrodes developed recently for water photoelectrolysis, can
also be used as efficient catalysts for degrading pollutants.
MECHANISM
In a photooxidative degradation process, an
n-type semiconductor is illuminated with radiation having energy at least equal
to the band-gap (Eg) of the semiconductor. The photons absorbed excite electrons
from the valence band (VB) to the conduction band (CB), while holes are left in
the valence band. The holes in a semiconductor have a high oxidizing power and
can oxidize organic molecules in an aqueous environment. These photoelectrons
and photoholes may recombine producing thermal energy, but they may be separated
and engaged in driving chemical reactions in photochemical processes. The rate
of oxidation by holes has to be balanced by the rate of the reduction by
electrons. The transfer of photogenerated holes from the valence band to organic
molecules may be either isoenergetic or inelastic via band-gap surface states.
Surface states can act as the recombination centers for photogenerated
electron-hole pairs but may also participate in sub-bandgap excitation processes
and help utilize more energy from the solar spectrum.
Bandgap
excitation in nanostructured semiconductor film
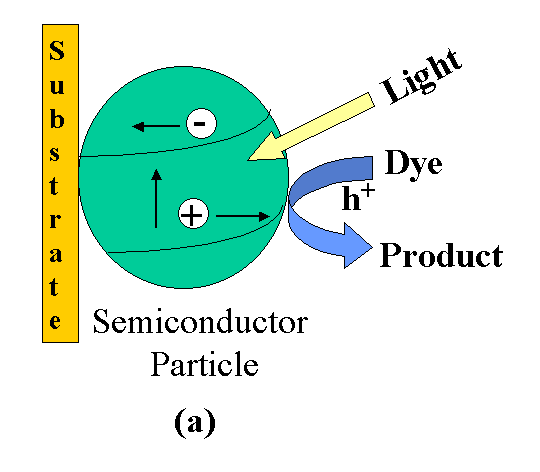
Figure 2.
Figure 3.
n-WO3
PHOTOELECTRODES
Tungsten trioxide is an important
photoelectrocatalytic material. The photoelectrochemical behavior of WO3 films
was extensively studied in this laboratory for electrochromic applications
[8,9], solar energy conversion, and degradation of pollutants. The
electrodeposited Pt/WO3 catalysts have also been found to have high activity
toward oxidation of methanol and formic acid, higher than benchmark Pt
catalysts. The WOx films with Pt, Sn, and Ru centers were used by Bock and
MacDougall for electrooxidation of HCOOH and (COOH)2. Kulesza and Faulkner
described electrocatalysis of chlorate reduction on mixed valency WO3-x
electrodes. The electrocatalytic activity of Pt/WO3 electrode in phosphoric acid
fuel-cells was studied by Savadogo. Augustynski et al., investigating
photooxidation of methanol on thermal WO3 films, have found that at high
methanol concentrations, hole scavenging is the predominant process while at low
methanol concentration, an indirect photooxidation with the formation of
hydrogen peroxide species takes place. Modulation of absorbance spectra of
composite films WO3-TiO2 were described by de Tacconi et al. The photoelectrodes were synthesized by pulsed electrodeposition. Krasnov and
Kolbasov have found that index of refraction of electrodeposited a-WO3 films
increases during the film drying process, indicating on chemisorbed water
content.
AZO DYE
Remazol Black B (RBB) is a complex textile diazo
dye and has a high photo- and thermal stability. It cannot be efficiently
degraded using conventional methods of oxidative degradation such as ozonation.
Recently, a ZnO-assisted photocatalytic degradation of RBB has been reported.
Various metal oxide semiconductors were used by Poulios and Tsachpinis in
their RBB photodegradation investigations. The decolorization of RBB on TiO2 and CdS photocatalysts in aqueous suspensions was also investigated. Reaction
mechanisms for reduction of several diazo dyes on mercury electrode were
elucidated by Zanoni and coworkers. Biodegradability studies of Ganesh et
al. have shown that RBB and other azo dyes are not biodegradable under aerobic
conditions.
 Remazol Black B structure
Remazol Black B structure
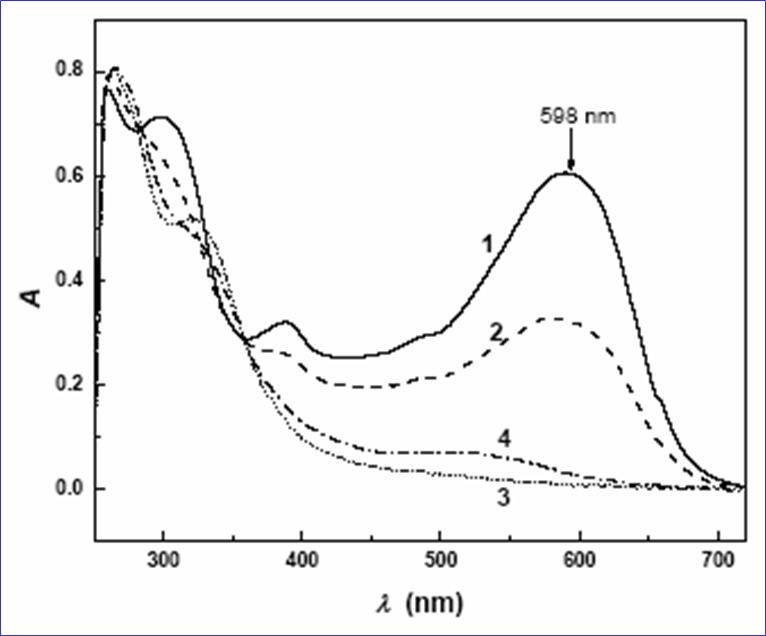
Figure 4. UV-VIS spectra of RBB recorded during dye degradation
process.
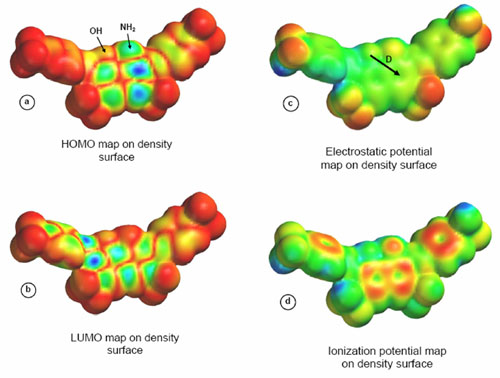
Figure 5. Electronic structure of RBB.
Back to Student/Faculty Research
Department of Chemistry



