
Department of Chemistry, SUNY-Potsdam Slide 1 2 3 4 5 6 7 8 9 10
Presented at: <<< >>>
12th International Conference on Electroanalysis ESEAC-2008
Prague, Czech Republic, June 16-19, 2008

| Symposium | |
|
|
"ENVIRONMENTAL ASPECTS OF GSH REDOX REGULATION AND OXIDATIVE STRESS", M. Hepel, J. Dallas, Department of Chemistry, State University of New York at Potsdam, Potsdam, NY |
| Abstract | |
|
The
redox regulating glutathione (GSH) system plays a vital role in living
organisms in protecting cells against oxidative damage. There are
indications that susceptibilities of different individuals to
environmentally induced diseases (diabetes, brain damage and cancer) are
associated with GSH level in cells and body fluids. The interactions of GSH
with various toxicants are suspected to influence the regulating capacity of
GSH/GSSG system. We have investigated GSH interactions with toxic heavy
metal ions1,2 on electrodes using electrochemical quartz crystal
nanogravimetry and immittance spectroscopy. W report here on Hg(II)-GSH
interactions. We have found that the modification of a Au substrate with
self-assembled glutathione (Au-SG) film creates a framework of
confined-space microenvironment with ion-channels for enhanced Hg2+-GSH
interactions and rich charge transfer reactivity. The reduction of Hg(II) on
a Au-SG piezoelectrode has been investigated in two regimes of ion channel
permeation of the modifying film, at opened and closed ion channels. The
maximum surface coverage determined from upd-Hg mass:
qHg
= 0.31. The chelation of Hg2+ to carboxylate moieties at the
outer film-solution boundary and place–exchange of Hg/Au atoms at the
sulphur root of adsorbed GSH have been further investigated3 by
ab initio quantum mechanical calculations.
The electronic structure of Hg2+-GSH chelate
was found to mimic that of Ca2+-GSH chelate. The implications of
this finding for studies of environmental effects on susceptibility to
degenerative diseases should be of great significance.
REFERENCES
1.
M. Hepel, E. Tewksbury, J. Electroanal. Chem., 552, 291 (2003).
2. M. Hepel, E. Tewksbury, Electrochim. Acta,
49, 3827 (2004).
3.
M. Hepel, J. Dallas, in press. |
|
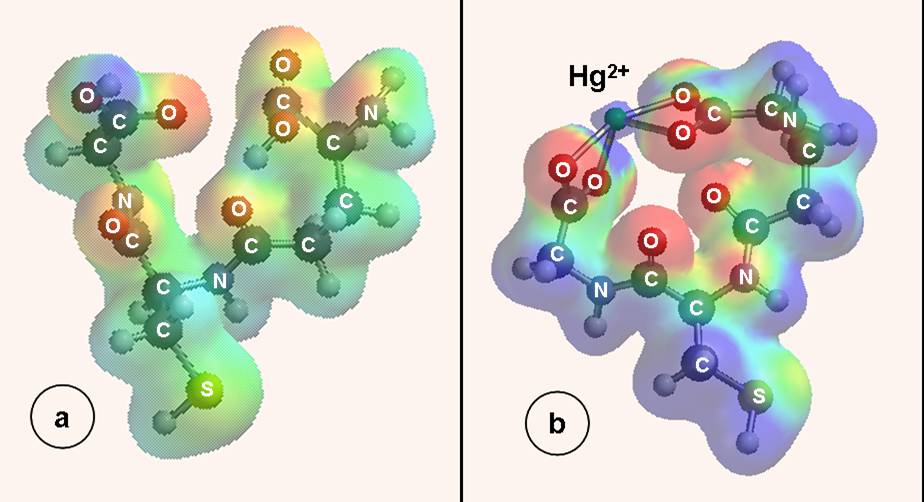
| Fig. 1. Electron density surfaces for: (a) glutathione (GSH) and (b) GSH-Hg2+ surface complex.in a GSH-SAM on Au piezoelectrode. |