Division
of Inorganic Chemistry, Symposium: INOR
Division
of Inorganic Chemistry, Symposium: INORDepartment of Chemistry, SUNY-Potsdam Slide 1 2 3 4 5 6 7 8 9 10
Presented at:
<<< >>>The National Meeting of the American Chemical Society
Boston, MA, August 19-23, 2007

 Division
of Inorganic Chemistry, Symposium: INOR
Division
of Inorganic Chemistry, Symposium: INOR
Haley Redmond and Maria Hepel, Department of Chemistry, State University of New York at Potsdam, 44 Pierrepont Ave., Potsdam, NY 13676, hepelmr@potsdam.edu
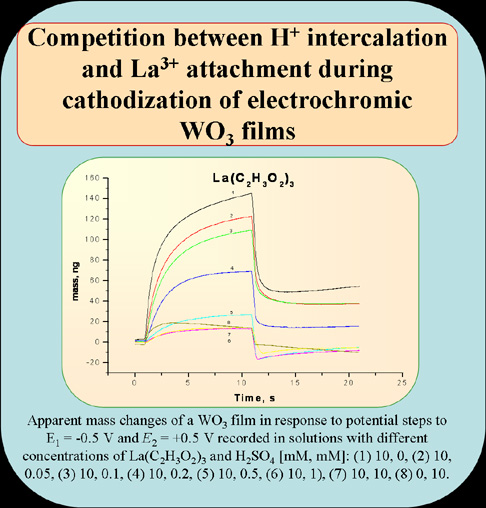
Abstract. Electrochromic properties and optical density modulation in WO3 films have been studied to elucidate the mechanism of reversible ion intercalation and formation of color centers. The intercalation and electrostatic attachment of inorganic cations and alkyl ammonium cations to WO3 nanoparticles have been investigated using Electrochemical Quartz Crystal Nanobalance (EQCN) and spectroscopic techniques. Strong dependence of the cation ingress on the degree of cation hydration in aqueous solutions has been found. The competition of metal cation intercalation with the uptake of hydronium ions by WO3 films has been observed. The limited mass increases for some cations have been attributed to local water dissociation and hydronium ion uptake. These processes result in pH increase and WO3 reduction, the strongest effect being observed in the presence of alkyl ammonium cations. Improvements in the electrooptic switching performance of WO3 films have been achieved by shortening the solid-state diffusion paths and nanostructured film design.
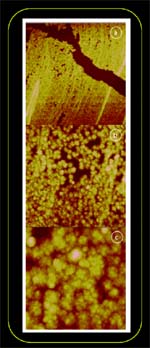
 AFM images of WO3 films and switching
characteristics
AFM images of WO3 films and switching
characteristics
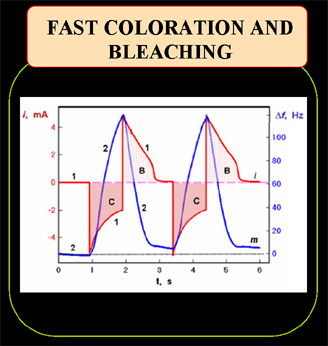
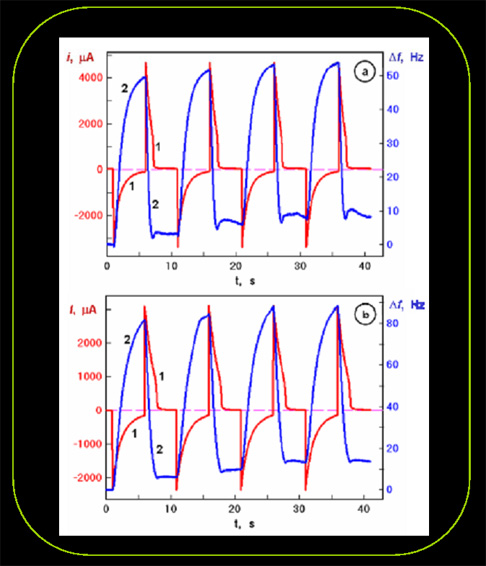 Current and mass transients during optical switching
Current and mass transients during optical switching