 THE DEPARTMENT OF AT STATE
UNIVERSITY OF NEW YORK, POTSDAM N.Y.
THE DEPARTMENT OF AT STATE
UNIVERSITY OF NEW YORK, POTSDAM N.Y.CHEMISTRY
Research with
Students
Students List
Learning through research
Raman Imaging and Spectroscopy Lab
Chemical and Biological Applications of Raman Spectroscopy (pdf, 7 MB)
Quartz Crystal Nanobalance Lab
Studies
Electrochemical Quartz Crystal Nanobalance (EQCN) technique utilizes quartz vibrations and piezoelectric effect to measure mass changes as small as a fraction of a monolayer of atoms
Back to
Professor Maria Hepel
EQCN Lab
Stowell Hall
44 Pierrepont Ave.
Potsdam , NY 13676, U.S.A.
Tel.: +1.315.267.2267
Fax: +1.315.267.3170
The Electrochemical Quartz Crystal Nanobalance (EQCN) is a measurement system for monitoring extremely small variation in the mass of a working electrode attached to a vibrating quartz single crystal. We use thin wafers cut from a quartz crystal at specific orientation (35015' angle) and with precise thickness (0.166 mm), which oscillate at a characteristic fundamental frequency of 10 MHz. The working electrode is in the form of a thin metal film evaporated on one side of a quartz crystal wafer, which is then sealed to the side opening in an electrochemical cell. Any change in the mass rigidly attached to the working electrode results in the change of the quartz crystal oscillation frequency. The frequency of the working quartz crystal is measured and compared to the frequency of the standard reference quartz crystal. Hence, the frequency measurements are differential, i.e. the frequency of the reference crystal is subtracted from the frequency of the working crystal. The obtained frequency difference is then converted to an analog voltage using a Frequency-to-Voltage Converter and measured using a 16-bit analog-to-digital converter (ADC). The frequency shift is proportional the mass change of working electrode.
Typical processes leading to the frequency change which corresponds to the effective mass change at the working electrode are listed below:
DNA hybridization & defective gene detection
DNA - drug interactions
antigen - antibody binding
adsorption/desorption
metal/alloy plating
surface oxidation
corrosion and corrosion protection
etching
heterogeneous polymerization
ion ingress/egress from ion exchange films
oxidation/reduction of conductive polymers
coadsorption and competitive adsorption
intercalation (lithium batteries, electrochromic materials)
gas sensors and explosives detection
etc.
With the Model EQCN-700, it is possible to monitor time transients of the effective electrode mass change in an electrochemical or non-electrochemical cell filled with liquid or gas. Voltammetric experiments of any type can be performed simultaneously with monitoring mass transients.
The resolution of the EQCN-700 is 0.01 Hz which corresponds approximately to 0.01 ng of the effective mass change. The exceptional linearity of mass measurements extends up to 100 μg. The use of AT-cut quartz crystals reduces temperature coefficient to the minimum. Under normal circumstances, the effect of temperature can be neglected in the range near the room temperature. If a very high sensitivity or wide temperature range are required, a thermostatted cell and high stability reference generator can be used. The reference oscillator in our system has the frequency stability of 0.000001 % (0..01 ppm). We use also a high stability external frequency source (Model FG-806), to adjust reference frequency in a wide range from 9.95 to 10.05 MHz to accommodate quartz crystal resonators with different frequencies.
In electrochemical measurements, a potentiostat is also required in the measurement setup. We use Models PS-205 and PS-605 precision potentiostats / galvanostats for exceptionally low noise and high precision.
The EQCN experiments are completely computer controlled with the Data Logger DAQ-716v with Real-Time VOLTSCAN Data Acquisition and Control System (based on a 16-bit D/A converter and 16-bit A/D converters). All the data processing, graphing and spreadsheet reporting is done with Voltscan 5.0 software. The experimental data can also be exported to common spreadsheet and graphics packages, such as the MS EXCEL, Microcal ORIGIN, etc.
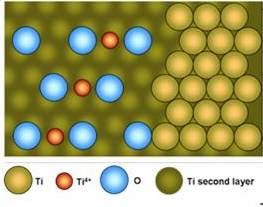
Nanoporous TiO2
for solar energy conversion and direct methanol fuel cells
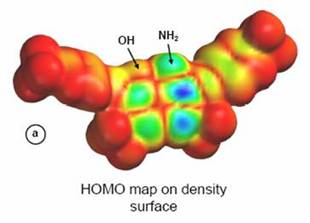
Degradation of dye pollutants
Electron density surface
with map of highest Occupied Molecular Orbitals (HOMO) for dye pollutant Remazol Blue Black. Decomposition of pollutants studied by photo-electrocatalytic method using TiO2, WO3, and MoO3 semiconductor electrodes
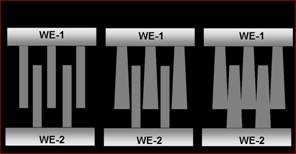
Quantum Conductance Monatomic Nanobridge Devices
studied using conductanc spectroscopy and AFM/STM
Model of a Phospholipid Bilayer Film with Gramicidin Molecular Channel
self-assmbled on a gold piezoelectrode in EQCN experiments
RESEARCH TEACHING STUDENTS PRESENTATIONS