 Symposium
of the
DIVISION of MEDICINAL CHEMISTRY
Symposium
of the
DIVISION of MEDICINAL CHEMISTRY

Department of Chemistry, SUNY-Potsdam Slide 1 2 3 4 5 6 7 8 9 10 <<< >>>
Presented at:
The National Meeting of the American Chemical Society
Boston, MA, August 22-26, 2010
 Symposium
of the
DIVISION of MEDICINAL CHEMISTRY
Symposium
of the
DIVISION of MEDICINAL CHEMISTRY

3. Electrochemical reactivity and interactions of quercetin with DNA in the presence of Cu(II) ions
Sara Cutler, Magdalena Stobiecka, and Maria Hepel, Department of Chemistry, State University of New York at Potsdam, 44 Pierrepont Ave., Potsdam, NY 13676, Fax: 315-267-3170, hepelmr@potsdam.edu
|
Abstract. Quercetin is one of the most abundant molecules of the flavonoid group and affords many beneficial effects on human health, including cardiovascular protection, anticancer, anti-allergy, and antiviral activity. Although, it has been known to have anti-inflammatory and anti-oxidant properties, recent studies indicate that under certain conditions quercetin may act as a prooxidant and may induce oxidative DNA damage in the presence of transition metal ions. To evaluate the mutagenic activity of quercetin and its carcinogenic potential, we have investigated the interactions of quercetin with DNA and Cu(II) ions. The changes in DNA structure induced by the triple complex formation were investigated by monitoring the guanosine oxidation peak of DNA at glassy carbon electrode. The quercetin-DNA adduct formation was analyzed using the electrochemical quartz crystal nanobalance (EQCN). The interactions of quercetin with DNA and Cu(II) ions were also investigated in solution by resonance elastic light scattering (RELS) and UV-Vis absorbance spectroscopy.
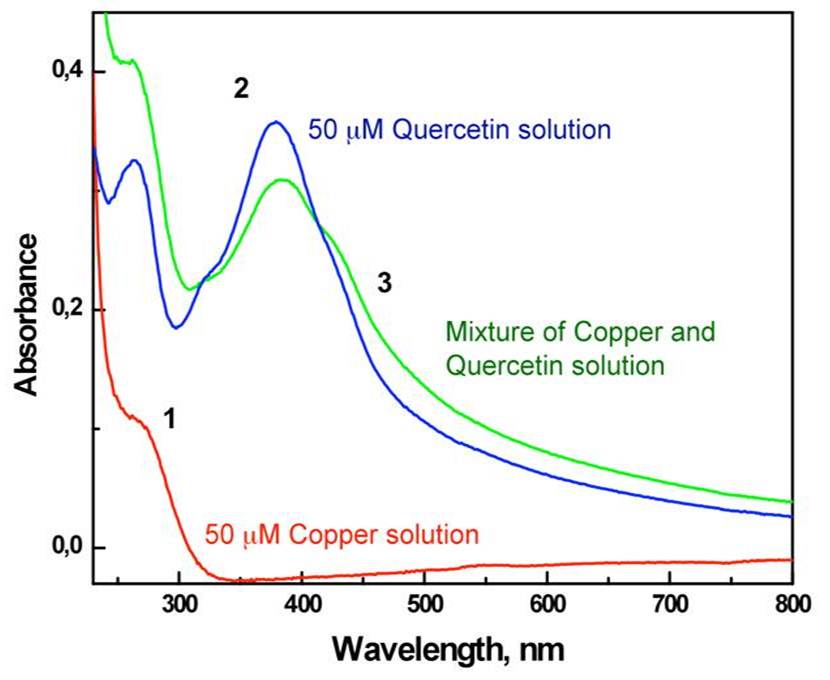
Figure 1. Absorbance spectra of 50 uM quercetin solutions in the absence (2) and in the presence (3) of a 50 uM Cu(II). |
 |
|
Back to Student/Faculty Research Department of Chemistry