 Symposium
of the
DIVISION of PHYSICAL CHEMISTRY
Symposium
of the
DIVISION of PHYSICAL CHEMISTRY

Department of Chemistry, SUNY-Potsdam Slide 1 2 3 4 5 6 7 8 9 10 <<< >>>
Presented at:
The National Meeting of the American Chemical Society
Boston, MA, August 22-26, 2010
 Symposium
of the
DIVISION of PHYSICAL CHEMISTRY
Symposium
of the
DIVISION of PHYSICAL CHEMISTRY

6. Novel ligand-exchange technique for rapid functionalization of monolayer-protected gold nanoparticles
Zachary Reed, Robert Wallace, Sara Cutler, Magdalena Stobiecka, and Maria Hepel, Department of Chemistry, State University of New York at Potsdam, 44 Pierrepont Ave., Potsdam, NY 13676, Fax: 315-267-3170, hepelmr@potsdam.edu
|
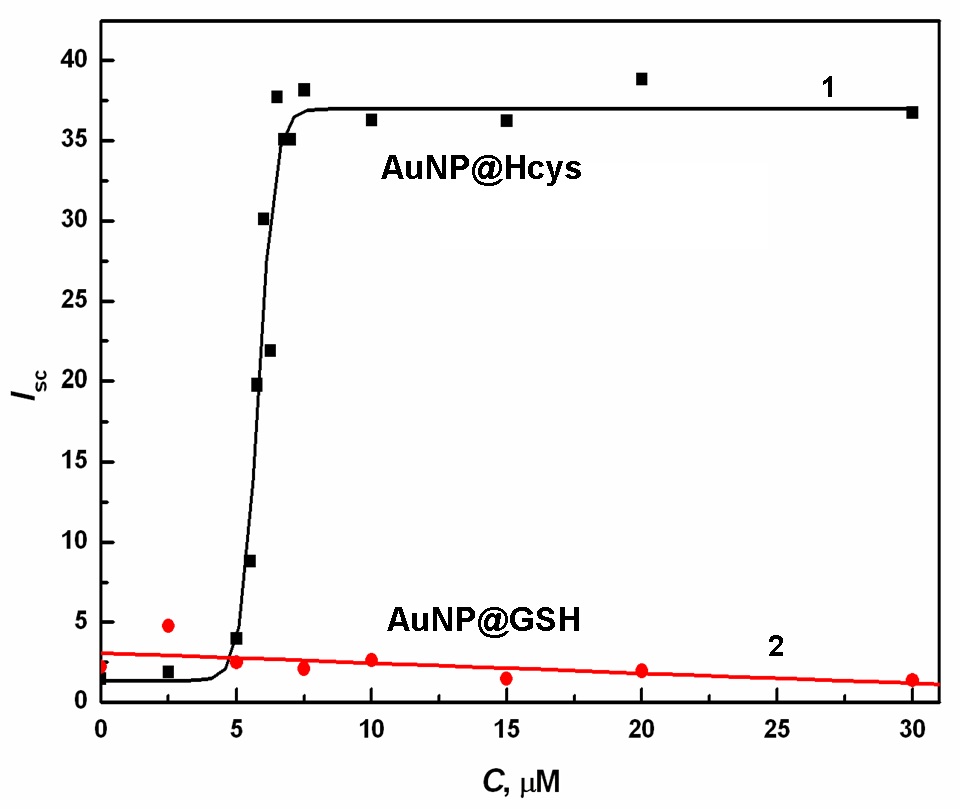
Abstract. A new nanoparticle functionalization methodology based on a fast one-step ligand-exchange has been developed. The fine-tuning of the film composition, which is the key element of this technique, was achieved by utilizing moderator molecules able to control the interfacial ligand-exchange process. Since the ligand-exchange process on Au terraces is inherently slow then to attain high exchange rates, we have developed a new strategy based on the sequential place-exchange process starting from nucleation sites. The nucleation of ligand exchange domains occurs at the edge defects in a basal film followed by the domain growth until a complete film conversion is attained. This model represents an alternative approach to the approach considering the ligand-exchange as a random place-exchange process proceeding evenly on the entire SAM surface. The experimental evaluation performed using resonance elastic light scattering spectroscopy and utilizing homocysteine as the incoming ligand and glutathione as the moderator is presented.
Figure 1. Dependence of RELS intensity Isc on concentration of: (1) GSH, (2) Hcys, for a solution of citrate-capped AuNP5nm; CAuNP = 3.8 nM, CCit = 0.46 mM, pH = 5, λex = 560 nm; t = 60 s. |
 |
|
Back to Student/Faculty Research Department of Chemistry