 THE DEPARTMENT OF AT STATE
UNIVERSITY OF NEW YORK, POTSDAM N.Y.
THE DEPARTMENT OF AT STATE
UNIVERSITY OF NEW YORK, POTSDAM N.Y.CHEMISTRY
Research with
Students
List of Research Projects
Learning through research
Raman Imaging and Spectroscopy Lab
Chemical and Biological Applications of Raman Spectroscopy (pdf, 7 MB)
Quartz Crystal Nanobalance Lab
Studies
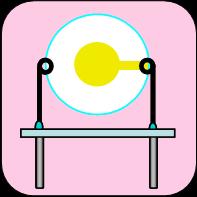
Electrochemical Quartz Crystal Nanobalance (EQCN) technique utilizes quartz vibrations and piezoelectric effect to measure mass changes as small as a fraction of a monolayer of atoms
Back to
Home Page
Professor Maria Hepel
hepelmr@potsdam.edu
Stowell Hall
44 Pierrepont Ave.
Potsdam , NY 13676, U.S.A.
Tel.: +1.315.267.2267
Fax: +1.315.267.3170

-
Dr. Hepel research studies focus on novel nanoscience phenomena in electron transport in atomically-thin nanowires, catalysis on nanaparticles and nanoporous materials, chemical quantum interference, interactions of submonolayer films with biomolecules, and applications of new discoveries in emerging nanotechnologies.
-
Her interest in incorporating interdisciplinary and nanoscience projects in undergraduate courses brings an exciting opportunity for students to learn skills and understanding of nanoscience for future careers in chemistry, biosensors, piezoimmunosensors for the detection of biomolecules, toxicants, and heavy metals, as well as in materials science, molecular electronics, and energy technologies, such as the fuel cells, solar photovoltaics, hydrogen energy, lithium batteries, electrochromic devices, etc.
-
The cutting-edge research introduces students to the state-of-the-art modern instrumentation, including Atomic Force Microscope, Scanning Tunneling Microscope, Quartz Crystal Nanogravimetric and Immittance Spectroscopic instruments, Ultra-Fast Electrochemical Potentiostats, Quantum Nanobridge Impedance Spectroscopy, and others.
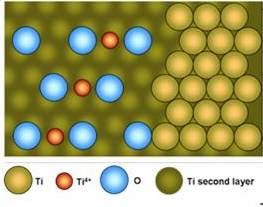
Nanoporous TiO2
for solar energy conversion and direct methanol fuel cells
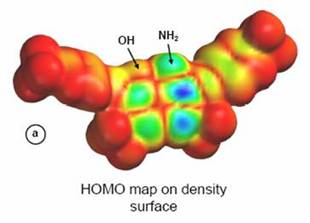
Degradation of dye pollutants
Electron density surface
with map of highest Occupied Molecular Orbitals (HOMO) for dye pollutant Remazol Blue Black. Decomposition of pollutants studied by photo-electrocatalytic method using TiO2, WO3, and MoO3 semiconductor electrodes
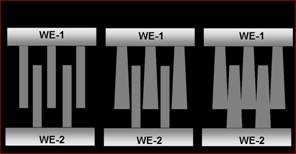
Quantum Conductance Monatomic Nanobridge Devices
studied using conductanc spectroscopy and AFM/STM
RESEARCH with STUDENTS TEACHING PRESENTATIONS